Toll-like receptor
From Wikipedia, the free encyclopedia
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single, membrane-spanning, non-catalytic receptors that recognize structurally conserved molecules derived from microbes. Once these microbes have breached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs which activates immune cell responses.
They receive their name from their similarity to the protein coded by the Toll gene identified in Drosophila in 1985 by Christiane Nüsslein-Volhard.[1] The name is not a reference to toll booths or tolls of any kind. The gene in question, when mutated, makes the Drosophila flies look unusual, or 'weird'. The researchers were so surprised that they spontaneously shouted out in German "Das ist ja toll!" which translates as "That´s amazing/great!".[2]
Contents[hide] |
[edit]Diversity
TLRs are a type of pattern recognition receptor (PRR) and recognize molecules that are broadly shared by pathogens but distinguishable from host molecules, collectively referred to as pathogen-associated molecular patterns (PAMPs). TLRs together with the Interleukin-1 receptors form a receptor superfamily, known as the "Interleukin-1 Receptor/Toll-Like Receptor Superfamily"; all members of this family have in common a so-called TIR (Toll-IL-1 receptor) domain.
Three subgroups of TIR domains exist. Proteins with subgroup 1 TIR domains are receptors for interleukins that are produced by macrophages, monocytes and dendritic cells and all have extracellularImmunoglobulin (Ig) domains. Proteins with subgroup 2 TIR domains are classical TLRs, and bind directly or indirectly to molecules of microbial origin. A third subgroup of proteins containing TIR domains consists of adaptor proteins that are exclusively cytosolic and mediate signaling from proteins of subgroups 1 and 2.
TLRs are present in vertebrates, as well as in invertebrates. Molecular building blocks of the TLRs are represented in bacteria and in plants, and in the latter kingdom, are well known to be required for host defence against infection. The TLRs thus appear to be one of the most ancient, conserved components of the immune system.
[edit]Discovery
When microbes were first recognized as the cause of infectious diseases, it was immediately clear that multicellular organisms must be capable of recognizing them when infected, and hence, capable of recognizing molecules unique to microbes. A large body of literature, spanning most of the last century, attests to the search for the key molecules and their receptors. More than 100 years ago,Richard Pfeiffer, a student of Robert Koch, coined the term "endotoxin" to describe a substance produced by Gram-negative bacteria that could provoke fever and shock in experimental animals. In the decades that followed, endotoxin was chemically characterized and identified as a lipopolysaccharide (LPS) produced by most Gram-negative bacteria. Other molecules (bacterial lipopeptides, flagellin, and unmethylated DNA) were shown in turn to provoke host responses that are normally protective. However, these responses can be detrimental if they are excessively prolonged or intense. It followed logically that there must be receptors for such molecules, capable of alerting the host to the presence of infection, but these remained elusive for many years.
Toll-like receptors are now counted among the key molecules that alert the immune system to the presence of microbial infections. They are named for their similarity to Toll, a receptor first identified in the fruit fly Drosophila melanogaster, and originally known for its developmental function in that organism. In 1996, Toll was found by Jules A. Hoffmann and his colleagues to have an essential role in the fly's immunity to fungal infection,[3] which it achieved by activating the synthesis of antimicrobial peptides.
The first reported human Toll-like receptor was described by Nomura and colleagues in 1994,[4] mapped to a chromosome by Taguchi and colleagues in 1996.[5] Because the immune function of Toll inDrosophila was not then known, it was assumed that TIL (now known as TLR1) might participate in mammalian development. However, in 1991 (prior to the discovery of TIL) it was observed that a molecule with a clear role in immune function in mammals, the interleukin-1 (IL-1) receptor, also had homology to drosophila Toll; the cytoplasmic portions of both molecules were similar.[6]
In 1997, Charles Janeway and Ruslan Medzhitov showed that a Toll-like receptor now known as TLR4 could, when artificially ligated using antibodies, induce the activation of certain genes necessary for initiating an adaptive immune response.[7]. TLR 4 function as an LPS sensing receptor was discovered by Bruce A. Beutler and colleagues.[8] These workers used positional cloning to prove that mice that could not respond to LPS had mutations that abolished the function of TLR4. This identified TLR4 as one of the key components of the receptor for LPS.
In turn, the other TLR genes were ablated in mice by gene targeting, largely in the laboratory of Shizuo Akira and colleagues. Each TLR is now believed to detect a discrete collection of molecules of microbial origin, and to signal the presence of infections.
[edit]Extended family
It has been estimated that most mammalian species have between ten and fifteen types of Toll-like receptors. Thirteen TLRs (named simply TLR1 to TLR13) have been identified in humans and mice together, and equivalent forms of many of these have been found in other mammalian species.[9][10][11] However, equivalents of certain TLR found in humans are not present in all mammals. For example, a gene coding for a protein analogous to TLR10 in humans is present in mice, but appears to have been damaged at some point in the past by a retrovirus. On the other hand, mice express TLRs 11, 12, and 13, none of which are represented in humans. Other mammals may express TLRs which are not found in humans. Other non-mammalian species may have TLRs distinct from mammals, as demonstrated by TLR14, which is found in the Takifugu pufferfish.[12] This may complicate the process of using experimental animals as models of human innate immunity.
[edit]Ligands
Because the specificity of Toll-like receptors (and other innate immune receptors) cannot easily be changed in the course of evolution, these receptors recognize molecules that are constantly associated with threats (i.e. pathogen or cell stress) and are highly specific to these threats (i.e. cannot be mistaken for self molecules). Pathogen-associated molecules that meet this requirement are usually critical to the pathogen's function and cannot be eliminated or changed through mutation; they are said to be evolutionarily conserved. Well conserved features in pathogens include bacterialcell-surface lipopolysaccharides (LPS), lipoproteins, lipopeptides and lipoarabinomannan; proteins such as flagellin from bacterial flagella; double-stranded RNA of viruses or the unmethylated CpGislands of bacterial and viral DNA; and certain other RNA and DNA. For most of the TLRs, ligand recognition specificity has now been established by gene targeting (also known as "gene knockout"): a technique by which individual genes may be selectively deleted in mice.[13][14] See the table below for a summary of known TLR ligands.
[edit]Endogenous ligands
The stereotypic inflammatory response provoked by TLR activation has prompted speculation that endogenous activators of TLRs might participate in autoimmune diseases. TLRs have been suspected of binding to host molecules including fibrinogen (involved in blood clotting) and heat shock proteins (HSPs)and host DNA.
[edit]Signaling
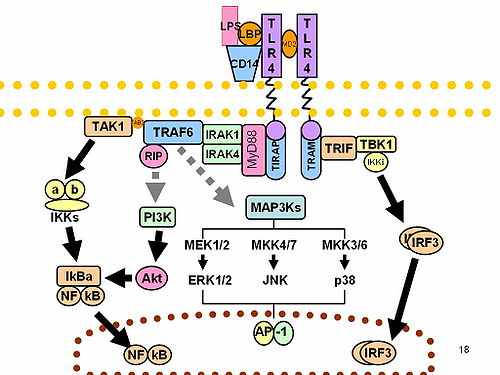
TLRs are believed to function as dimers. Though most TLRs appear to function as homodimers, TLR2 formsheterodimers with TLR1 or TLR6, each dimer having a different ligand specificity. TLRs may also depend on other co-receptors for full ligand sensitivity, such as in the case of TLR4's recognition of LPS, which requires MD-2. CD14 and LPS Binding Protein (LBP) are known to facilitate the presentation of LPS to MD-2.
The adapter proteins and kinases that mediate TLR signaling have also been targeted. In addition, random germline mutagenesis with ENU has been used to decipher the TLR signaling pathways. When activated, TLRs recruit adapter molecules within the cytoplasm of cells in order to propagate a signal. Four adapter molecules are known to be involved in signaling. These proteins are known as MyD88, Tirap (also called Mal),Trif, and Tram.[15][16][17] The adapters activate other molecules within the cell, including certain protein kinases (IRAK1, IRAK4, TBK1, and IKKi) that amplify the signal, and ultimately lead to the induction or suppression of genes that orchestrate the inflammatory response. In all, thousands of genes are activated by TLR signaling, and collectively, the TLRs constitutes one of the most pleiotropic yet tightly regulated gateways for gene modulation.
[edit]Summary of known mammalian TLRs
Toll-like receptors bind and become activated by different ligands, which, in turn are located on different types of organisms or structures. They also have different adapters to respond to activation and are located sometimes at the cell surface and sometimes to internal cell compartments. Furthermore, they are expressed by different types of leucocytes or other cell types:
| Receptor | Ligand(s) [18] | Ligand location [18] | Adapter(s) | Location | Cell types[18] | |
|---|---|---|---|---|---|---|
| TLR 1 | multiple triacyl lipopeptides | Bacteria | MyD88/MAL | cell surface |
| |
| TLR 2 | multiple glycolipids | Bacteria | MyD88/MAL | cell surface |
| |
| multiple lipopeptides | Bacteria | |||||
| multiple lipoproteins | Bacteria | |||||
| lipoteichoic acid | Bacteria | |||||
| HSP70 | Host cells | |||||
| zymosan (Beta-glucan) | Fungi | |||||
| Numerous others | ||||||
| TLR 3 | double-stranded RNA, poly I:C | viruses | TRIF | cell compartment |
| |
| TLR 4 | lipopolysaccharide | Gram-negative bacteria | MyD88/MAL/TRIF/TRAM | cell surface |
| |
| several heat shock proteins | Bacteria and host cells | |||||
| fibrinogen | host cells | |||||
| heparan sulfate fragments | host cells | |||||
| hyaluronic acid fragments | host cells | |||||
| Numerous others | ||||||
| TLR 5 | flagellin | Bacteria | MyD88 | cell surface |
| |
| TLR 6 | multiple diacyl lipopeptides | Mycoplasma | MyD88/MAL | cell surface |
| |
| TLR 7 | imidazoquinoline | small synthetic compounds | MyD88 | cell compartment |
| |
| loxoribine (a guanosine analogue) | ||||||
| bropirimine | ||||||
| single-stranded RNA | ||||||
| TLR 8 | small synthetic compounds; single-stranded RNA | MyD88 | cell compartment |
| ||
| TLR 9 | unmethylated CpG Oligodeoxynucleotide DNA | Bacteria | MyD88 | cell compartment |
| |
| TLR 10 | unknown | unknown | unknown | cell surface |
| |
| TLR 11 | Profilin | Toxoplasma gondii | MyD88 | cell surface |
| |
| TLR 12 | unknown | unknown | ? |
| ||
| TLR 13 | unknown | unknown | ? |
| ||
| TLR 15 | unknown | Salmonella enterica serovar Typhimurium[21] | unknown | ? | ? |
[edit]Activation and effects
Following activation by ligands of microbial origin, several reactions are possible. Immune cells can produce signalling factors called cytokines which trigger inflammation. In the case of a bacterial factor, the pathogen might be phagocytosed and digested, and its antigens presented to CD4+ T cells. In the case of a viral factor, the infected cell may shut off its protein synthesis and may undergo programmed cell death (apoptosis). Immune cells that have detected a virus may also release anti-viral factors such as interferons.
The discovery of the Toll-like receptors finally identified the innate immune receptors that were responsible for many of the innate immune functions that had been studied for many years. Interestingly, TLRs seem only to be involved in the cytokine production and cellular activation in response to microbes, and do not play a significant role in the adhesion and phagocytosis of microorganisms.
[edit]Drugs interactions
Imiquimod (cardinally used in dermatology), and its successor resiquimod, are ligands for TLR7 and TLR8.[22]
The lipid A analogon eritoran acts as a TLR4 antagonist. As of December 2009, it is being developed as a drug against severe sepsis.[23]
[edit]References
- ^ Hansson GK, Edfeldt K (2005). "Toll to be paid at the gateway to the vessel wall". Arterioscler. Thromb. Vasc. Biol. 25 (6): 1085–7. doi:10.1161/01.ATV.0000168894.43759.47. PMID 15923538.
- ^ www.aerzteblatt.de/archiv/55316/
- ^ Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA (September 1996). "The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults". Cell 86 (6): 973–83. doi:10.1016/S0092-8674(00)80172-5. PMID 8808632.
- ^ Nomura N, Miyajima N, Sazuka T, et al. (1994). "Prediction of the coding sequences of unidentified human genes. I. The coding sequences of 40 new genes (KIAA0001-KIAA0040) deduced by analysis of randomly sampled cDNA clones from human immature myeloid cell line KG-1" ([dead link] – Scholar search). DNA Res. 1 (1): 27–35. doi:10.1093/dnares/1.1.27. PMID 7584026.
- ^ Taguchi T, Mitcham JL, Dower SK, Sims JE, Testa JR (March 1996). "Chromosomal localization of TIL, a gene encoding a protein related to the Drosophila transmembrane receptor Toll, to human chromosome 4p14". Genomics 32 (3): 486–8. doi:10.1006/geno.1996.0150. PMID 8838819.
- ^ Gay NJ, Keith FJ (May 1991). "Drosophila Toll and IL-1 receptor". Nature 351 (6325): 355–6. doi:10.1038/351355b0. PMID 1851964.
- ^ Medzhitov R, Preston-Hurlburt P, Janeway CA (July 1997). "A human homologue of the Drosophila Toll protein signals activation of adaptive immunity". Nature 388 (6640): 394–7. doi:10.1038/41131.PMID 9237759.
- ^ Poltorak A, He X, Smirnova I, et al. (December 1998). "Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene". Science (journal) 282 (5396): 2085–8. PMID 9851930.
- ^ Du X, Poltorak A, Wei Y, Beutler B (September 2000). "Three novel mammalian toll-like receptors: gene structure, expression, and evolution". Eur. Cytokine Netw. 11 (3): 362–71. PMID 11022119.
- ^ Chuang TH, Ulevitch RJ (September 2000). "Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9". Eur. Cytokine Netw. 11 (3): 372–8. PMID 11022120.
- ^ Tabeta K, Georgel P, Janssen E, et al. (March 2004). "Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection". Proc. Natl. Acad. Sci. U.S.A. 101 (10): 3516–21. doi:10.1073/pnas.0400525101. PMID 14993594. PMC 373494.
- ^ Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A (2005). "The evolution of vertebrate Toll-like receptors". Proc Natl Acad Sci USA 102 (27): 9577–9582.doi:10.1073/pnas.0502272102. PMID 15976025. PMC 1172252.
- ^ Hoebe K, Du X, Georgel P, et al. (August 2003). "Identification of Lps2 as a key transducer of MyD88-independent TIR signalling". Nature 424 (6950): 743–8. doi:10.1038/nature01889. PMID 12872135.
- ^ Hemmi H, Takeuchi O, Kawai T, et al. (December 2000). "A Toll-like receptor recognizes bacterial DNA". Nature 408 (6813): 740–5. doi:10.1038/35047123. PMID 11130078.
- ^ Shigeoka AA, Holscher TD, King AJ, et al. (May 2007). "TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways". J. Immunol. 178 (10): 6252–8. PMID 17475853.
- ^ Yamamoto M, Sato S, Hemmi H, et al. (November 2003). "TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway". Nat. Immunol. 4 (11): 1144–50.doi:10.1038/ni986. PMID 14556004.
- ^ Yamamoto M, Sato S, Hemmi H, et al. (November 2002). "Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4". Nature 420 (6913): 324–9. doi:10.1038/nature01182.PMID 12447441.
- ^ a b c Unless else specified in boxes then ref is: Waltenbaugh C, Doan T, Melvold R, Viselli S (2008). Immunology. Lippincott's Illustrated reviews. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 17. ISBN 0-7817-9543-5.
- ^ a b c d Sallusto F, Lanzavecchia A (2002). "The instructive role of dendritic cells on T-cell responses". Arthritis Res. 4 Suppl 3: S127–32. doi:10.1186/ar567. PMID 12110131.
- ^ a b Mishra BB, Gundra UM, Teale JM (2008). "Expression and distribution of Toll-like receptors 11-13 in the brain during murine neurocysticercosis". Journal of Neuroinflammation 5: 53.doi:10.1186/1742-2094-5-53. PMID 19077284. PMC 2631477.
- ^ Higgs R, Cormican P, Cahalane S, Allan B, Lloyd AT, Meade K, James T, Lynn DJ, Babiuk LA, O'farrelly C (2006). "Induction of a novel chicken Toll-like receptor following Salmonella enterica serovar Typhimurium infection". Infection and Immunity 74 (3): 1692–1698. doi:10.1128/IAI.74.3.1692–1698.2006. PMID 16495540. PMC 1418683.
- ^ Peter Fritsch (2004) (in German). Dermatologie Venerologie : Grundlagen. Klinik. Atlas.. Berlin: Springer. ISBN 3-540-00332-0.
- ^ Tidswell, M; Tillis, W; Larosa, SP; Lynn, M; Wittek, AE; Kao, R; Wheeler, J; Gogate, J et al. (2010). "Phase 2 trial of eritoran tetrasodium (E5564), a Toll-like receptor 4 antagonist, in patients with severe sepsis". Critical care medicine 38 (1): 72–83. doi:10.1097/CCM.0b013e3181b07b78. PMID 19661804.
[edit]External links
- MeSH Toll-Like+Receptors
- TollML: Toll-like receptors and ligands database at University of Munich
- Toll-Like Receptor Signaling Pathway
- The Toll-Like Receptor Family of Innate Immune Receptors (pdf)
- Toll-Like receptor Pathway
Imiquimod
From Wikipedia, the free encyclopedia
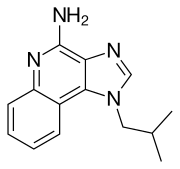 | |
|---|---|
| Systematic (IUPAC) name | |
| 3-(2-methylpropyl)-3,5,8-triazatricyclo[7.4.0.02,6]trideca-1(9),2(6),4,7,10,12-hexaen-7-amine | |
| Identifiers | |
| CAS number | 99011-02-6 |
| ATC code | D06BB10 |
| PubChem | CID 57469 |
| DrugBank | APRD01030 |
| Chemical data | |
| Formula | C14H16N4 |
| Mol. mass | 240.304 g/mol |
| SMILES | eMolecules & PubChem |
| Synonyms | 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine |
| Pharmacokinetic data | |
| Half-life | 30 hours (topical dose), 2 hours (subcutaneous dose) |
| Therapeutic considerations | |
| Licence data | |
| Pregnancy cat. | B1(AU) C(US) |
| Legal status | POM (UK) ℞-only (US) |
| Routes | Topical |
| | |
Imiquimod (INN) is a prescription medication that acts as an immune response modifier. It is marketed by MEDA AB, Graceway Pharmaceuticals and iNova Pharmaceuticals under the trade name Aldara and by Mochida as Beselna.
Contents[hide] |
[edit]History
The original FDA approval was on February 27, 1997, FDA Application No. (NDA) 020723, by 3M. Imiquimod is approved to treat actinic keratosis, superficial basal cell carcinoma, and external genital warts. Adverse side effects have been reported, in some cases serious and systemic, resulting in the revision of warning labels.
[edit]Uses
Imiquimod is a patient-applied cream used to treat certain diseases of the skin, including skin cancers (basal cell carcinoma, Bowen's disease,[1] superficialsquamous cell carcinoma, some superficial malignant melanomas, and actinic keratosis) as well as genital warts (Condylomata acuminata). It has also been tested for treatment of molluscum contagiosum, vulvar intraepithelial neoplasia, common warts that have proven difficult to treat, [2] and vaginal intraepithelial neoplasia.[3] Outstanding cosmetic result has resulted from the treatment of both large superficial basal cell carcinoma and squamous cell carcinoma in-situ, but the morbidity and discomfort of the treatment can be severe. Focal recurrence of tumor has been seen after imiquimod treatment, but appear to be amenable to surgical excision.
Imiquimod can also cause subclinical lesions to become visible. This unmasking effect is felt to be of clinical benefit as lesions that may have otherwise have been missed are being treated. Photographs of actinic keratosis and basal cell carcinomas before, during and after treatment show the unmasking of subclinical disease.[4]
[edit]Mechanism of action
The exact mechanism of action in which imiquimod and its analogs activate the immune system is not yet known. Nevertheless, it is known that imiquimod activates immune cells through the toll-like receptor 7 (TLR7), commonly involved in pathogen recognition, on the cell surface.[5] Cells activated by imiquimod via TLR-7 secrete cytokines (primarily interferon-α (IFN-α), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α)).[6] There is evidence that imiquimod, when applied to skin, can lead to the activation of Langerhans cells, which subsequently migrate to local lymph nodes to activate the adaptive immune system.[7] Other cell types activated by imiquimod include natural killer cells, macrophages and B-lymphocytes.[7]
New research has shown that imiquimod's anti-proliferative effect is totally independent of immune system activation or function. Imiquimod exerts its effect by increasing levels of the opioid growth factor receptor (OGFr). Blocking OGFr function with siRNA technology resulted in loss of any antiproliferative effect of imiquimod.[8]
[edit]Disadvantages
| This section needs additional citations for verification. Please help improve this article by adding reliable references. Unsourced material may be challenged and removed. (May 2009) |
Non specific inflammation and dermatitis can occur during use of imiquimod for genital warts and molluscum.[citation needed] This often occurs where the skin is traumatized from scratching, or between skin folds. Blisters, bloody dry eschar, pain and discomfort often follows the use of imiquimod for skin cancers and precancerous growths.[citation needed] During the treatment of large superficial basal cell carcinoma or squamous cell cancer in situ, areas of black dried crust often form.[citation needed] Many individuals with extensive actinic keratosis cannot tolerate the resulting reaction either.[citation needed] Fortunately, after completion of the therapy, the skin often heals with barely any scarring.
Recurrence of skin cancer has been noted with imiquimod, but often appears to be localized. It is more common when there are deeply penetrating nests of tumor cells such as in nodular basal cell carcinoma. Recurrence can be treated surgically by local excision. The recurrence rate depends on the condition being treated and the frequency of topical imiquimod application. A 6-week study on 99 patients with superficial basal cell carcinomas found success rates of 100%, 88%, 73% and 70% for twice daily, once daily, 6 times weekly and 3 times weekly application, respectively.[9]
Other side effects include headaches, back pain, muscle aches, tiredness, flu-like symptoms, swollen lymph nodes, diarrhea, and fungal infections.[10]
[edit]See also
[edit]Chemistry
Gertser, J. F.; 1985, EP patent 0145340 .
[edit]References
- ^ van Egmond S, Hoedemaker C, Sinclair R (2007). "Successful treatment of perianal Bowen's disease with imiquimod". Int J Dermatol 46 (3): 318–9. doi:10.1111/j.1365-4632.2007.03200.x.PMID 17343595.
- ^ van Seters M, van Beurden M, ten Kate FJ, et al. (April 2008). "Treatment of vulvar intraepithelial neoplasia with topical imiquimod". The New England journal of medicine 358 (14): 1465–73.doi:10.1056/NEJMoa072685. PMID 18385498.
- ^ Buck HW, Guth KJ (October 2003). "Treatment of vaginal intraepithelial neoplasia (primarily low grade) with imiquimod 5% cream". Journal of lower genital tract disease 7 (4): 290–3.doi:10.1097/00128360-200310000-00011. PMID 17051086.
- ^ Photographs before, during, and after imiquimod therapy for actinic keratosis and basal cell carcinoma
- ^ Hemmi, H., et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol.. 2002 3(2):196-200. PMID 11812998.
- ^ Sauder, D.N., Imiquimod: modes of action. British Journal of Dermatology. 2003 149(Suppl. 66):5-8. PMID 14616337
- ^ a b Miller, R.L., et al. Imiquimod applied topically: a novel immune response modifier and a new class of drug. Int J Immunopharmacol. 1999 Jan;21(1):1-14. PMID 10411278
- ^ Zagon IS, Donahue RN, Rogosnitzky M, McLaughlin PJ (August 2008). "Imiquimod upregulates the opioid growth factor receptor to inhibit cell proliferation independent of immune function". Exp. Biol. Med. (Maywood) 233 (8): 968–79. doi:10.3181/0802-RM-58. PMID 18480416.
- ^ Advances in the use of topical imiquimod to treat dermatologic disorders
- ^ Aldara website, What are the possible side effects of Aldara Cream?
[edit]External links
Resiquimod
From Wikipedia, the free encyclopedia
 | |
|---|---|
| Systematic (IUPAC) name | |
| 1-[4-amino-2-(ethoxymethyl)imidazo[4,5-c]quinolin-1-yl]-2-methylpropan-2-ol | |
| Identifiers | |
| CAS number | 144875-48-9 |
| ATC code | None |
| PubChem | CID 159603 |
| Chemical data | |
| Formula | C17H22N4O2 |
| Mol. mass | 314.382 g/mol |
| SMILES | eMolecules & PubChem |
| Therapeutic considerations | |
| Pregnancy cat. | B1(AU) C(US) |
| Legal status | POM (UK) ℞-only (US) |
| Routes | Topical |
Resiquimod (R-848) is a drug that acts as an immune response modifier, and has antiviral and antitumour activity. It is used as a topical cream in the treatment of skin lesions[1] such as those caused by herpes simplex virus,[2][3] and as an adjutant to increase the effectiveness of vaccines.[4] It has several mechanisms of action, being both an agonist for toll-like receptor 7 and 8,[5] and an upregulator of the opioid growth factor receptor.[6]
[edit]See also
[edit]References
- ^ Szeimies RM, Bichel J, Ortonne JP, Stockfleth E, Lee J, Meng TC (July 2008). "A phase II dose-ranging study of topical resiquimod to treat actinic keratosis".The British Journal of Dermatology 159 (1): 205–10. doi:10.1111/j.1365-2133.2008.08615.x. PMID 18476957.
- ^ Wu JJ, Huang DB, Tyring SK (November 2004). "Resiquimod: a new immune response modifier with potential as a vaccine adjuvant for Th1 immune responses". Antiviral Research 64 (2): 79–83. doi:10.1016/j.antiviral.2004.07.002. PMID 15498602.
- ^ Fife KH, Meng TC, Ferris DG, Liu P (February 2008). "Effect of resiquimod 0.01% gel on lesion healing and viral shedding when applied to genital herpes lesions". Antimicrobial Agents and Chemotherapy 52 (2): 477–82. doi:10.1128/AAC.01173-07. PMID 18039918.
- ^ Tomai MA, Miller RL, Lipson KE, Kieper WC, Zarraga IE, Vasilakos JP (October 2007). "Resiquimod and other immune response modifiers as vaccine adjuvants". Expert Review of Vaccines 6 (5): 835–47. doi:10.1586/14760584.6.5.835. PMID 17931162.
- ^ Hurst J, Prinz N, Lorenz M, Bauer S, Chapman J, Lackner KJ, von Landenberg P (February 2009). "TLR7 and TLR8 ligands and antiphospholipid antibodies show synergistic effects on the induction of IL-1beta and caspase-1 in monocytes and dendritic cells". Immunobiology 214 (8): 683–91.doi:10.1016/j.imbio.2008.12.003. PMID 19249118.
- ^ Zagon IS, Donahue RN, Rogosnitzky M, McLaughlin PJ (August 2008). "Imiquimod upregulates the opioid growth factor receptor to inhibit cell proliferation independent of immune function". Experimental Biology and Medicine (Maywood, N.J.) 233 (8): 968–79. doi:10.3181/0802-RM-58. PMID 18480416.



No comments:
Post a Comment